Draw a Water Molecule and Indicate Its Polarity
This molecule is nonpolar. Therefore water is said to be a polar molecule which means that there is an uneven distribution of electron density.
Lesson Overview 2 2 Properties Of Water Ppt Video Online Download
It also depicts how a charge such as on an ion Na or Cl for example can interact with a water molecule.

. Hydrogen has one proton and one electron. The OCS molecule has a structure similar to CO 2 but a sulfur atom has replaced one of the oxygen atoms. Do I draw these 2 compounds separately and Im unsure of how.
Draw a bohr diagram of the protons and electrons in each of the following. Helps to elucidate its importance in maintaining life. Draw in dipole arrows for all polar covalent bonds starting the arrow at the more electropositive atom and ending at the more electronegative atom.
From in between the hydrogen atoms to the oxygen atom. Although C and S have very similar electronegativity values S is slightly more electronegative than C and so the C. Water is polar due to the uneven sharing of electrons between oxygen and hydrogen.
B In contrast water is polar because the OH bond moments do not cancel out. CCl 2 F 2 d. To determine if this molecule is polar we draw the molecular structure.
The value obtained is 61709 10 30 C. Water is polar in nature. Since oxygen has more protons it would attract electrons more strongly.
_ Why is water considered a. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative. The absolute values of the electronegativity differences between the atoms in the bonds HH HCl and NaCl are 0 nonpolar 09 polar covalent and 21 ionic respectively.
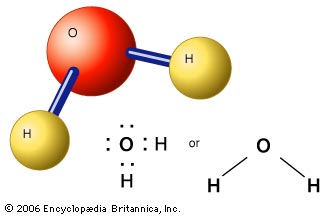
Waters chemical formula is H 2 O. CCl 2 F 2. First draw the Lewis electron dot diagram for water and determine its molecular shape.
The C-O bond is considerably polar. CF 2 H 2 e. At the molecular level salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar with positive and negative.
The electron pair geometry of SCl 4 is trigonal bipyramidal but there are 4 bonds and one lone pair in an equatorial position. Lower density as a solid water Ice is the solid form of water and solidifies and floats when the temperature drops to 0 degrees Celsius. There are 4 bonds all identical with zero lone pairs on Si.
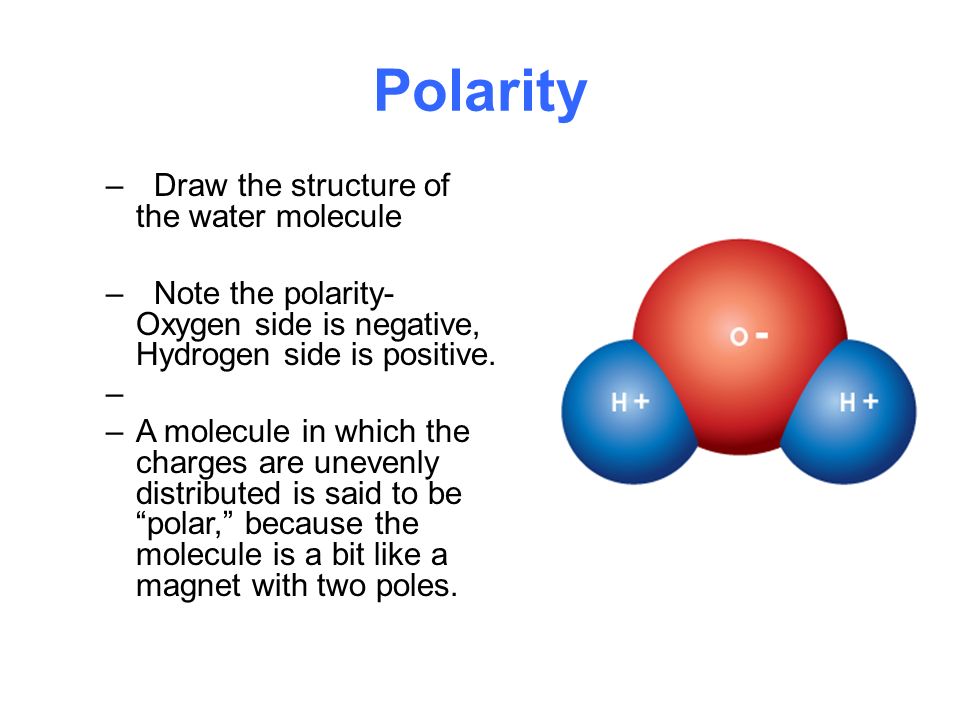
Oxygen has 8 protons where hydrogen has 1. Use that information to draw a diagram of a water molecule that shows electron sharing between the single oxygen and the two hydrogens. This means that even though the molecule as a whole is neutral the oxygen side has a slight negative charge while the hydrogen side has a slight positive charge.
The hydrogen and oxygen within water molecules H2O form polar covalent bonds. Looking at the electronegativity and shape of the H_2O molecule tells you how the arrow depicts the polarity. See explanation We start by looking at a water molecule.
To determine if this molecule is polar we draw the molecular structure. This creates a partial charge called a dipole. Polarity of water depends on the electronegativity difference between the atoms and the molecular shape of water.
First draw the Lewis structures. VSEPR theory predicts a linear molecule. Indicate if SiCl 4 and SCl 4 are polar or nonpolar.
Water at 20C will be 100177 cm 3 g -1. As temperature increase or decrease from 4C the volume occupied by one gram of water increases. Chemistry - Lewis Dots.
Water is an example of a polar solvent one of the best capable of dissolving most other compounds because of the water molecules unequal distribution of charge. The covalent bonds are therefore polar and the oxygen atoms have a slight negative charge from the presence extra electron share while the hydrogens are slightly positive from the extra un-neutralized protons. The shape of SiCl 4 is tetrahedral.
So if polarity of water is 185 D then 185 333564 10 30 will be its value in Cm. VSEPR theory predicts a linear molecule. Water H2O should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond.
When it is large the bond is polar covalent or ionic. Water has four electron groups but only two atoms attached to the central atom so it is bent. Connect the dipole arrows tail-to-head.
When the difference is very small or zero the bond is covalent and nonpolar. CH 4 tetrahedral non-polar b. Draw the molecule by placing atoms on the grid and connecting them with bonds.
Waters Polarity One of waters important properties is that it is composed of polar molecules. N 2 O i. Draw a water molecule and indicate its polarity.
Thus oxygen end becomes negative and hydrogen end becomes positive. NCl 3 trigonal pyramidal polar c. In solution the weak positively charged side of one water molecule will be attracted to the weak negatively charged side of another water molecule and the two molecules will be held together by what is called a.
The imbalance of charges in a water molecule results in water being a polar molecule. This diagram shows the positive and negative parts of a water molecule. _ Honors Biology Date.
View Notes - Water from SCIENCE Biology Ho at Charlottesville High. H 2 O m. It is least at 4C.
Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules. Thus at 0C water will have a volume of 100012 cm 3 g -1 and ice will be 109 cm 3 g -1. The ionic compounds lithium fluoride LIF and beryllium chloride Becl2.
As we can see the 2 hydrogen atoms are covalently bonded to the oxygen atom which has two lone pairs 4 total electrons that push. Oxygen has 8 protons 8 neutrons and 8 electrons. Then draw the structural formula.
Consequently the electrons in the water molecule spend slightly more time around the oxygen atomic center and less time around the hydrogen atomic centers. This molecule is polar. Water is most dense at about 4C and less dense above and below 4C.
The C-O bond is considerably polar. CH 2 O f. There are two lone pairs of electron.

Lesson Summary Water And Life Article Khan Academy

The Structure And Properties Of Water Introduction To Chemistry
How Do We Draw The Dipole Moment Of A Water Molecule Socratic
No comments for "Draw a Water Molecule and Indicate Its Polarity"
Post a Comment